Patient Reported Outcomes: let’s keep patients engaged as key stakeholder in R&I
Have you ever heard about Patient Reported Outcomes; do you know what they are?
Patient Reported Outcomes (PROs) are:
“Any outcome evaluated directly by the patient him/herself and based on patient’s perception of a disease and its treatment(s)” (European Medicines Agency, 2014).
PROs are of utmost importance to the research & healthcare community for many reasons, such as research outcomes evaluation (e.g. use of PROs in clinical trials or observational studies), Research & Innovation (R&I) impact assessment, but also in healthcare for monitoring of disease progression and evolution.
Moreover, PROs are a core example of patient engagement. Although there is still much room for improvement towards a truly participatory approach in the design of the measures themselves, which are right now settled mainly in a top-down approach by clinicians. So far, PROs have mainly been used in post-marketing observational studies, though now they are increasingly used as secondary or tertiary outcomes in clinical trials on disease-modifying therapies and symptomatic treatments, whereas in rehabilitation trials are used as primary or co-primary outcomes. In this context, the integration of PROs with e-Health will be crucial, in the next years electronic PROs (ePRO) will be available. This revolution will allow to collect discrete data on self-perception of a disease and improve the usability of PROs in clinical and research areas.
The importance that PRO has gained in the last years is also highlighted by the many emerging initiatives in the field, with examples in the field of Multiple Sclerosis (MS): The Patient Reported Outcomes Initiative for MS (PROMS)[1] or the iConquerMS. There are also several databases at national level, such us the PROMOPRO-MS database, promoted by the Italian MS Society.
Thus, can we use PROs to maximize the impact of R&I?
We did it in MULTI-ACT, creating the Patient Reported Dimension (PRD).
Let’s see what exactly is the PRD…
MULTI-ACT proposes a co-accountability approach[2] in which conventional metrics related to the excellence dimension are integrated with new measures related to the economic (efficiency), social, efficacy (achieving the mission), and patient reported dimensions, resulting in the MULTI-ACT Master Scorecard[3].
The Patient-Reported Dimension (PRD) is a transversal dimension applied throughout the four dimensions of the MULTI-ACT model for enabling the Science of Patient Input [4], where PROs are investigated as metrics able to measure the impact of R&I on outcomes that matter most to patients.
The PRD includes indicators that are reported by patients, family and caregivers addressing functional reported aspects (i.e. PRO) and psychosocial reported aspects (i.e. metrics to evaluate the Return on Engagement – RoE). The indicators can be a collection of answers to questionnaires and active and/or passive data collection without the intervention of clinicians (e.g. eHealth via App/ICT devices like wearables or electronic bracelets). The main feature of the PRD is that it reports the perspective of the patient (PROs, RoE) or provides continued objective data (eHealth), therefore it’s not influenced by the clinician.
MULTI-ACT co-accountability focuses on the PROs (functional reported aspects) as it foresees the development of PROs as key indicators of impact, instrumental to enable a multi-stakeholder approach and effective patient engagement. Also psychosocial aspects (RoE) are important, and there is indeed the need to develop effective indicators.
A concrete example of functional reported aspects?
Take for example Multiple Sclerosis (MS), the case study of MULTI-ACT. The functional domains that matter most to people affected by MS are: Quality of Life and Satisfaction, Anxiety and Depression, Fatigue, Upper-limb dexterity, Locomotion, Cognitive function, Bladder function. Thus, PROs suitable to evaluate the impact of R&I on the outcomes that matter to them are indicators able to assess: patients’ satisfaction with their quality of life(Life Satisfaction Index and Neuro-QoL – Quality of Life in Neurological Disorders), their level of upper-limb dexterity (Abilhand – Manual ability for adults with upper limb impairment), their level of Anxiety and Depression (HADS – Hospital Anxiety and Depression Scale), their level of bladder function (OAB-Q – Overactive Bladder Questionnaire), their level of motor, cognitive, psycho-social fatigue(M-FIS – Modified-Fatigue-Impact-Scale, their level of locomotion (Twelve Item MS Walking Scale – MSWS-12). Click here for more info.
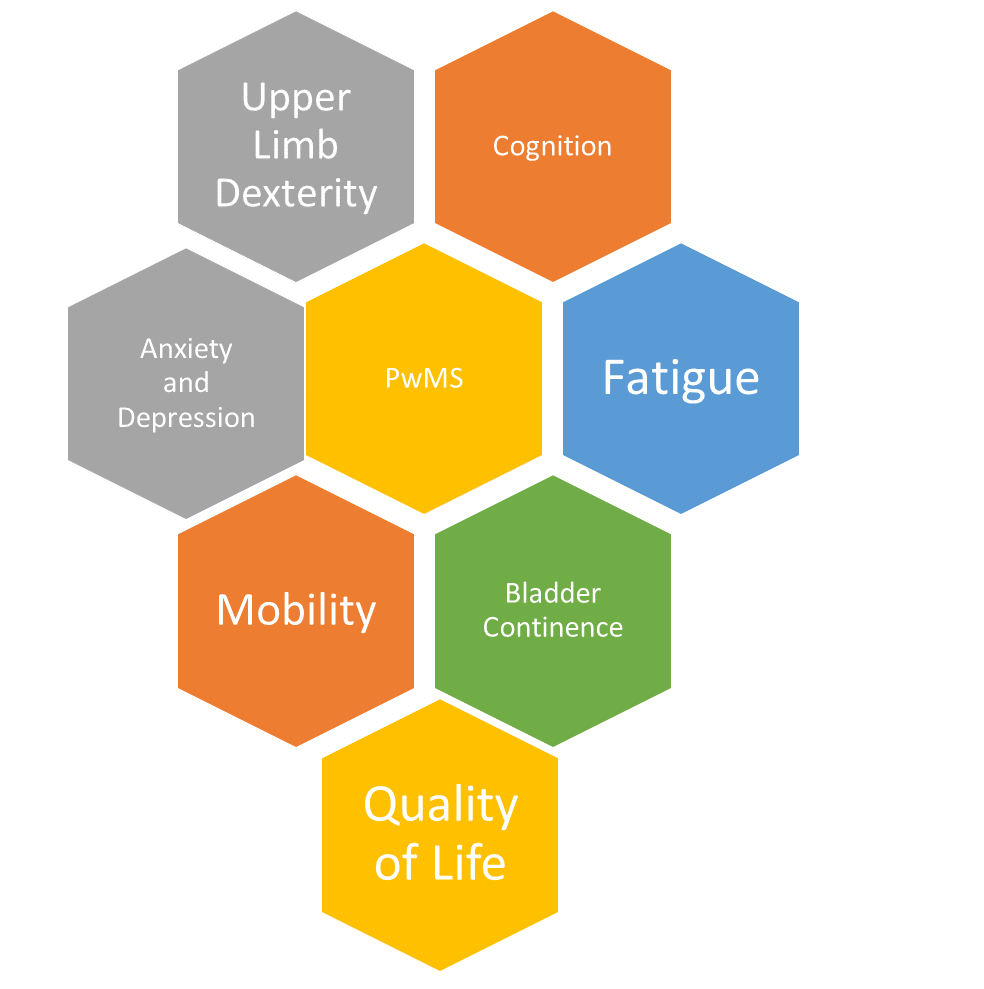
Functional domains that matter to people with MS (PwMS)
In fact, there is consensus in the clinical and scientific community that the classical clinical scales that are used to classify the level of disability in Multiple Sclerosis, such as the long-established Expanded Disability Status Scale (EDSS: a clinical scale used to classify level of disability in Multiple Sclerosis from 0 (no disability) to 9.5 (restricted to bed)), but also the Multiple Sclerosis Functional Composite Score (MSFC: is a composite score based on a walking performance test; a cognitive test and an upper limb performance test) ) are inadequate to capture the change of the patients’ clinical condition. See here for more info.
And what about psychosocial reported aspects?
Following a Public Consultation performed under MULTI-ACT activities and its Patient Engagement Guidelines, a selection of indicators that can be used for assessing the Return on Patient Engagement (RoE) have been identified to assess both the performance of patient engagement (i.e. the success of the initiative in terms of participation) and its effectiveness (i.e. the success of the initiative in term of real impact of the participation on the research process). An example of indicators for psychosocial reported aspects is presented below:
- Relevance of research to patients: The analysis of whether patients’ expectation with respect to the research and mission of the initiative are met;
- Patient endorsement: Endorsements given by patients to research activities and results;
- Patient satisfaction with the engagement: Patients’ expectation and satisfaction for and with their engagement in research.
But, why is PRD so important?
The PRD is of utmost importance to evaluate the impact of R&I on outcomes that matter most to patients and keep patients and stakeholders engaged along the R&I continuum.
MULTI-ACT co-accountability focuses on the PROs (functional reported aspects) as it foresees the development of PROs as key indicators of impact, instrumental to enable a multi-stakeholder approach and effective patient engagement. The fact that PROs are scientifically validated measures reported by the patient (final beneficiary of the health research) capture the interest of all the stakeholders. Indeed, PROs are able to focus on the needs of the final beneficiary of R&I and also to capture outcomes that correspond to the needs of all the other stakeholders.
We would love to hear your thoughts! If you wish, please email us a statement: multiact@braincouncil.eu. We are curious to hear from your society. Comments will be used in the further steps of MULTI-ACT development.
——————————————————————————————————————–
This blog was written by: Deborah Bertorello, Giampaolo Brichetto, Paola Zaratin.
[1] To know more on the initiative read: Measuring outcomes that matter most to people with multiple sclerosis: the role of patient-reported outcomes
[2] This blog entry is a direct continuation of the previous blog, “What is co-accountability and why is it important for multi-stakeholder initiatives?”. Read it here
[3] The Master Scorecard (MSC) is an adaptive tool for the application of the co-accountability model and its five dimensions. The MSC consists of a detailed list of indicators evaluating aspects of measurement linked to the different dimensions that can be tailored into different contexts and missions.
[4] “Science OF patient inputs” occurs when data of people with a disease are used (active and passive contribution) to evaluate the impact of R&I.


