Event Report: MULTI-ACT Final Conference
The MULTI-ACT Model: the path forward for participatory governance
in health research and innovation
The MULTI-ACT project, funded by the European Union’s Horizon 2020 Research and Innovation Programme, aimed to increase the impact of health research on people with brain conditions, creating and implementing a new model allowing for the effective cooperation of all relevant stakeholders.
On Tuesday, 23 March 2021, from 14.00 to 17.00, the MULTI-ACT project final conference took place with a view to showcase the work done and the results achieved over the past years by the project consortium.
Welcome and introduction
Dr. Paola Zaratin, Director of Scientific Research at the Italian Multiple Sclerosis Foundation (FISM) and Coordinator of the MULTI-ACT project, introduced the conference with a summary of the project, its funding, timeline, ambitions, and outcomes.
Dr. Zaratin underlined the need to ensure sustainability in brain research through new multi-stakeholder and multidisciplinary managerial models in line with the Responsible Research and Innovation (RRI) approach. She pointed out how the integration of RRI principles and models is particularly urgent in the field of brain conditions, as revealed by the COVID-19 pandemic, to enable a unique health research and care ecosystem through patient participation. She also highlighted the guiding principle of the MULTI-ACT model: “Act like an organisation and think like a movement”
Dr. Zaratin thanked the MULTI-ACT Consortium, the External Advisory Board, Patient Forum, and Patient Engagement Group, as well as other initiatives that have been collaborating and co-creating with the MULTI-ACT project. She concluded by saying that this good work and collaborations have enabled MULTI-ACT to be listed among other European RRI projects as a model expected to produce institutional changes in participatory research and innovation governance via innovative co-accountability strategy
Keynotes on Responsible Research and Innovation (RRI)
The conference was then kick-started by two keynote speeches addressing the ‘what’, ‘how’, and ‘why’ of Responsible Research and Innovation by Dr. René von Schomberg, European Commission, DG Research & Innovation, Open Science on Responsible Research & Innovation and Guest Professor at the Technical University Darmstadt; and Dr. Ralf Linder, Head of the Department Politics and Society and Coordinator for Technology Assessment and Governance within the Fraunhofer Institute for Systems and Innovation Research ISI.
Dr. René von Schomberg discussed the definition and rationale of ‘Open Science’, the difference with ‘closed science’, and how Open Science is linked to RRI. Dr. von Schomberg described the two types of crisis in science:
- Reproducibility crisis: science has become too competitive and close. Data is often shared at a later stage in the research process; much of the data is not reproducible; and data is generally not too robust.
- Productivity crisis: science is not driving to the expected outputs, which means that any investment in science is not proportionate to the outcomes obtained. This also relates to competitiveness and closedness.
These crises drove to a push for Open Science, where scientists start sharing their data as soon as possible with all knowledge stakeholders. The rationale of Open Science is that sharing knowledge and data as early as possible will facilitate the positive operationalisation of science. Open Science means openness to knowledge sources and to other actors in the research cycle; open outputs of science (e.g. data); and open knowledge producers. It makes science more efficient, reliable, and responsive to societal challenges. Open Science was proven critical and necessary in the management of the COVID-19 pandemic, but the question remains whether this applies only for emergencies and not in general.
Closed science is currently the most dominant system. In order to shift towards Open Science, instead of rewarding scientists for gaining scientific prestige, they should be rewarded for collaborating and sharing knowledge to achieve societal impact. Therefore, Open Science is a necessary, but insufficient condition for RRI.
One other important concept presented by Dr. von Schomberg was that RRI represents a new paradigm for innovation in which our social systems institutionalize collective co-responsibility as a driving force for socially desirable innovation by giving innovation a direction (mission-oriented approach) and, whenever possible, shaping its characteristics.
Dr. von Schomberg presented the different levels of reflexivity and normative approaches to RRI; placed the MULTI-ACT framework within those normative approaches; and provided resources to read more about RRI.
Dr. Ralf Linder opened his speech by discussing how the emergence of RRI is due to two key developments at the strategic policy level: the European Commission’s ambition in the early 2000s to better connect institutions with citizens, and the ongoing paradigm shift in science, technology, and innovation policy.
By way of background, Dr Linder described that since 1945, science, technology and innovation policy paradigms have gone from market to systemic failure, and now to directionality failure (focussing on grand challenges, mission-oriented innovation policy since the 2010s). Each paradigm is not replaced by the new one, instead they all co-exist, but with diminished weight and importance. What RRI is doing differently is democratising the science-society interface and addressing societal needs and challenges. This brings an emphasis on procedural dimensions, such as who defines the direction of Research & Innovation (R&I) and how; as well as how co-responsibility can be institutionalised.
At a national and international policy level, there is a strong presence of RRI (for example, in European funding schemes such as Horizon 2020), but insufficient translation into implementation. With regards to R&I institutions and practices, there is a low awareness of the policy concept of RRI, but a high degree of openness to and interest in responsibility ambitions. Additionally, in many institutions there are numerous RRI-related programmes in place, although they are not explicitly called RRI.
The conducive factors to the institutionalisation of RRI in any organisation are dedicated actors; external requirements, such as evaluations including RRI elements; a supportive internal incentive system; and the level of openness of the organisation to RRI. At the same time, there are also barriers and risks, such as a task overload, as RRI is time and resource consuming; a lack of resources, knowledge and capabilities of research organisations and researchers; organisational norms and values in conflict with RRI ambitions; the existing reward systems for academic careers; as well as, the risk that RRI becomes an exercise without meaningful transformation or practices.
Responsibility in research and innovation is an emergent process that is context specific. Research organisations must translate the abstract ideas of RRI to their own circumstances.
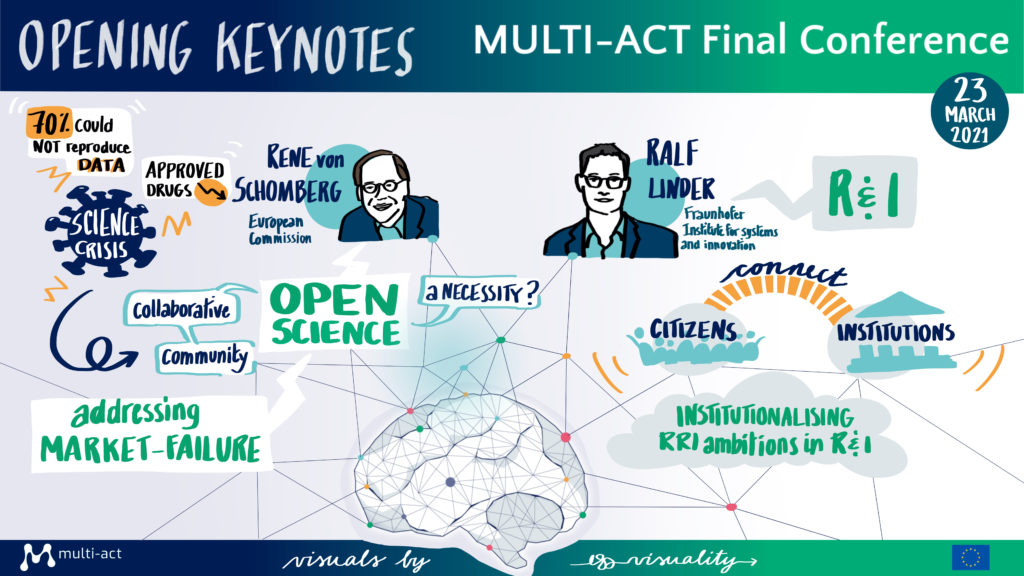
Panel discussion: ‘How relevant is the RRI approach in the health sector particularly in the context of emergencies such as the COVID-19 pandemic?’
The panel, moderated by Dr. Usman Khan, Senior Advisor at FIPRA, a European public affairs consultancy and the Good Governance Institute, consisted of three representatives of healthcare Research & Innovation: Dr. Elisabetta Verdun di Cantogno, Senior Medical Director at Global Affairs & Neuroscience, Merck Serono S.A; Prof. Giovanni Leonardi, Research Director at the Italian Health Ministry; and Dr. Robert McBurney, Chief Research Officer at iConquerMS Network.
RRI involves society as a whole and not just researchers; however, research has always been considered as far from society. This distance brought poor commitment from decision-makers – which can be seen in the financial commitment to R&I initiatives – but also research data not being accessible to citizens, and a consequential lack of trust and a difficulty to connect with the general public.
The COVID-19 pandemic has raised awareness on research; increased the commitment of the research community to practice open research; and brought different branches of medicine to unite and address both the direct impacts of COVID-19, but also its side effects. The pandemic showed the urgent need for more RRI initiatives and presented an opportunity to develop them. It also provided a single mission to health stakeholders: ‘manage COVID’, which facilitated collaboration. The emergency brought a change of mindset facilitating some practice changes in RRI, and there is hope to continue with some of these positive evolutions beyond the pandemic. Specifically, the scientific community has been leading the research and governments have had a supporting role in ensuring the success of R&I activities. Society at large, who were not fully aware of the crucial role of science, now recognise its pivotal role.
Another important aspect of RRI is partnerships. These are a dynamic process and, if progress has been made to ensure their effectiveness, more work remains to be done. A scientific community is a global network that exceeds national boundaries, and efforts should be directed to establish such networks and make them work effective. The Italian Ministry of Health joined one of these networks to follow the recommendations of Lancet in 2014, according to which research should meet the needs of patients; data should be shared; and there should be a sharing of good practices in health at the care level and not just at a scientific level. However, without the proper tools, co-designing with patients is difficult to achieve. In this perspective the Italian Ministry of Health decided to collaborate with the MULTI-ACT project, which provided tools that stakeholders could use in all fields of medical research, and not just brain disorders.
For several decades, organisations have been implementing initiatives based on responsible research and innovation, but have only recently started calling them ‘RRI’. Almost 20 years ago, iConquerMS Network faced some closedness in the implementation of their activities, and as a result, they created a biorepository; a patient-centred outcomes research institute which has helped researchers become more responsive to the need of citizens. With the increasing awareness on RRI, the unconscious competences of the organisation have become conscious ones.
Over the years, there has been a tremendous change in the Multiple Sclerosis (MS) and research community worldwide. Several initiatives on data sharing and patient-driven research were in the implementation process at the start of the pandemic, and this enabled rapid responses to meet the needs of MS patients and supporting communities when facing COVID-19. The pandemic has also helped the broad MS community to facilitate multi-stakeholder research, but it still took hard work and time to implement COVID-related research and build trust to bring forth the cultural change, although the governance framework was already there.
RRI also requires the pharmaceutical industry to pay more attention to the unmet needs and challenges of society. The pandemic was a prime example of how collaboration is necessary to meet and exceed the standard. Society is changing at a fast rate, but it remains crucial to consider the diversity of stakeholders and the impact of products being produced for each stakeholder group, thus working with inclusivity. It is also important to create the right instruments and work with patients from the beginning to prevent silos among researchers, care providers, and patients. Finally, there is a need to change the mindset, behaviours and approach to R&I; and there should be incentives to drive these changes.
During the pandemic, communications among and with the scientific community have been more rapid due to the increase use of digital technology. Similarly, the management of clinical trials has also been positively impacted by digital tools. Even direct industry competitors were collaborating at different extent, and stakeholders were working together to develop and design studies, thus facilitating rapid results. Stakeholders worked together to respond to COVID-19 and this generated improved partnerships and openness in research.
The RRI process is rich and more work is needed, but the COVID-19 pandemic has facilitated the implementation of RRI. Awareness of RRI among research and funding communities is still not as high as it could be, and other parallel activities are needed to raise awareness of different stakeholders. However, there is a widespread recognition of the importance of RRI, its implementation, and to reinvigorate the trust with the public.
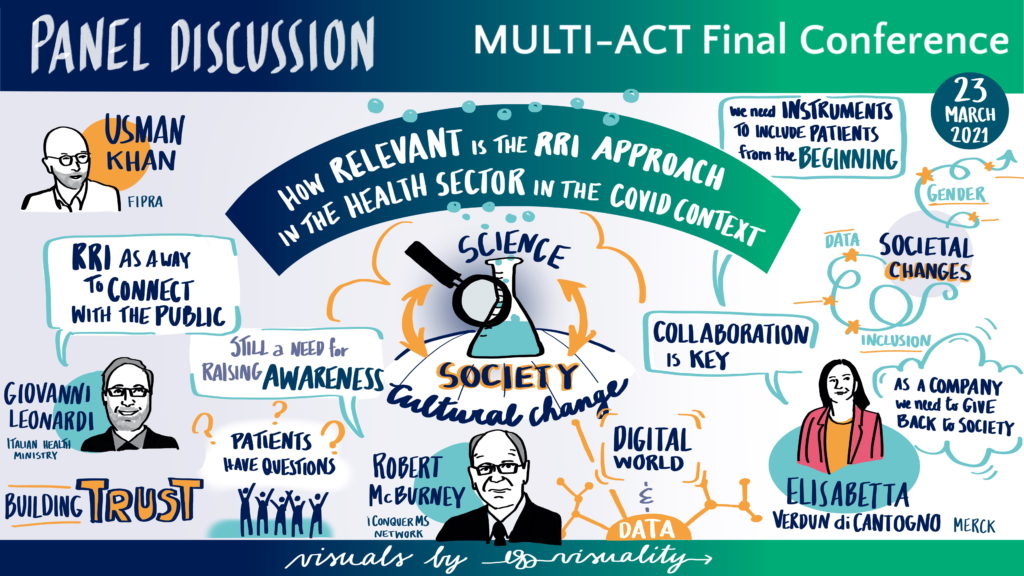
The MULTI-ACT Framework
In this session, members of the project consortium presented the MULTI-ACT framework.
Ms Valentina Tageo, Director of the International Projects Unit at ECHAlliance and project manager of the MULTI-ACT consortium, presented the process used to develop the MULTI-ACT Collective Research Impact Framework (CRIF); described the components of the framework and the link between the governance model and the impact assessment scorecard, and; presented the legacy of the project. Ms. Tageo explained how patients are involved in both the governance process and the impact assessment by presenting the concepts of ‘science with patient input’ and ‘science of patient input’. Finally, Ms. Tageo provided an overview of the user journey when the MULTI-ACT approach is used in R&I activities.
Ms Deborah Bertorello, International Project Manager at the Italian Multiple Sclerosis Foundation (FISM), discussed some of the guidelines that are core to the accountability approach of MULTI-ACT. With a view to fully engage patients and supporting communities in the governance of RRI, MULTI-ACT aims to capture the experiential knowledge of patients as a complementary approach to the expert patients’ knowledge. Understanding the experiential knowledge is necessary to measure outcomes that are important to patients; as such, the patient reported dimension in the Master Scorecard is transversal to all other dimensions. Furthermore, the biggest challenge in patient engagement is to ensure the representativeness of the community; and MULTI-ACT developed a new governance strategy to enable the involvement of professionals who are able to support the transition from individual to collective experiential knowledge. In this sense, MULTI-ACT empowers the patients’ ability to co-create.
Mr. Luca Molinari, Senior Consultant at Ernst & Young, presented the Materiality Analysis (MA) in the context of the project. The MA is a managerial tool increasingly viewed as a good practice in many companies. MULTI-ACT proposes a materiality analysis that allows stakeholders to sign a social contract (co-accountability); discover what aspects matter the most to their respective organisations; and find the right balance between the mission/agenda of an organisation and the outcome of the multi-stakeholder initiative. This approach is innovative, and the tool has given exceptional results. The challenge for organisations is often to identify the key stakeholders in doing the materiality analysis and to ensure their feedback are all considered. The MULTI-ACT digital toolkit provides a way to effectively collect and use that feedback.
Mr. Michele Andreaus, Professor at the University of Trento, presented the MULTI-ACT Master Scorecard. This is a flexible and customisable tool that can be tailored to different initiatives, research areas, diseases, and by a broad range of users. It includes a comprehensive set of indicators, but it is open to new ones. Prof. Andreus then presented how stakeholders of R&I initiatives can use the Master Scorecard starting from the five CRIF dimensions, to selecting the indicators that best fit their needs. To use the MULTI-ACT framework users do not need to have specific knowledge of the dimensions, all they need is a clear expectation of their return on engagement.
Dr. Sofia Tsekeridou, Senior Research and Innovation Manager-Expert at Intrasoft International, presented the Digital Toolbox, which has collected and aggregated all the know-how and tools developed by MULTI-ACT. The Digital Toolbox is user-friendly and it invites R&I initiatives to choose which aspects the Materiality Analysis to ask stakeholders; tracks responses by stakeholder groups; and provides final reports. The Digital Toolbox supports the development of a research implementation plan by facilitating stakeholders’ contribution to the design of the plan. Unsurprisingly, the most challenging part of developing the Toolbox was to ensure that all the feedback from stakeholders was properly integrated, which is exactly one of the challenges that the Toolbox aims to address.
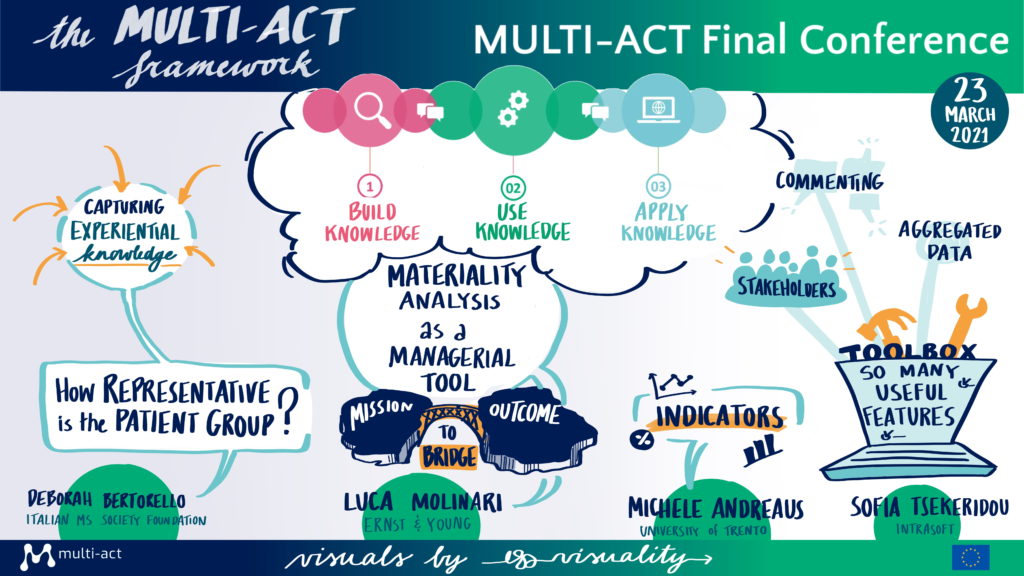
Interviews with MULTI-ACT CRIF Adopters
This session provided examples of how the MULTI-ACT model is being implemented.
Prof. David Henshall, Chair of EPICLUSTER, a cooperative action of epilepsy research coordinated by the European Brain Research Area, described the collaboration with MULTI-ACT. The collaboration took place during the first six months of the cluster, which at that time was missing key points in its governance, funding, and patient engagement. MULTI-ACT started by doing a ‘baseline analysis’ of the EPICLUSTER adherence to the MULTI-ACT Governance Model, thus highlighting both strengths and the rooms for improvement towards a stronger participatory governance and stakeholder engagement. The MULTI-ACT model also provided ways to implement changes, such as repairing some of the challenges in the governance and represented stakeholders. In addition, MULTI-ACT provided concrete suggestions for the development of an EPICLUSTER Patient Engagement Roadmap to find a way to engage scientific research with the needs of patients.
Mr. Graham McReynolds, Associate Vice President, Global Initiatives at the International Progressive MS Alliance. The Alliance is active in 19 different countries and includes diverse stakeholders such as Multiple Sclerosis organisations, researchers, clinicians, pharmaceutical companies, people with progressive MS and their supporting communities. The Alliance has been collaborating with MULTI-ACT to develop their research strategy while ensuring that all stakeholder perspectives were being considered worldwide. They believe that once everyone embraces RRI, the gap is to have a standard process, that is measurable, repeatable, and scientific, to involve people affected by MS in a stronger way. MULTI-ACT has helped collect the perspectives of patients from over 30 countries on what the research priority of the Alliance should be and will continue to collaborate with the Alliance to ensure that people affected by MS are engaging in all relevant decision-making stages of the research projects.
Prof. Giancarlo Comi, President of the European Charcot Foundation, presented how the MULTI-ACT framework is applied to the Multiple Sclerosis Care Unit (MSCU) Initiative to maximise and evaluate the impact of interdisciplinary care model for people with and affected by MS, with specific emphasis on those outcomes that matter to their patients. The European Charcot Foundation wanted to have a more comprehensive approach in MS research and bring together organisations working on the same objectives not to duplicate efforts. Therefore, they created the Multistakeholder Initiatives program fundamental to profile the activities of organisations following the life-course of patient with MS, including the MSCU project. The MSCU was selected as the case study of the MULTI-ACT project to test and apply the MULTI‐ACT CRIF into an existing multi‐stakeholder research initiative, at an early stage of development. The MULTI-ACT project met with MSCU representatives to discuss their governance, highlight opportunities for improvement to establish a structure with multi-stakeholder and accountable governance, and provide tailored recommendations. The co-creation activities performed with the MSCU led to a refined version of the MULTI-ACT Framework and the identification of several lessons learnt.
The panellists were unanimous in recommending potential users of the MULTI-ACT framework to test the project model on their initiatives and assess if the tools were able to define the return of investment for each of their stakeholders, and validate the framework in practice. If there may be scepticism from scientists in the value of the framework and worries on how it will change their practice, testing the MULTI-ACT model will be beneficial to prove that this is not a fad, but a change for the better in how research is being done.
The core to any multi-stakeholder engagement is trust. This requires trust in the process, but also that the involved stakeholders experience their perspectives being heard and acted upon. It also requires more time, discipline, and a new approach, to the point where it might even require a change in the R&I initiative’s agenda and mission. The MULTI-ACT model is a way to ensure that for research the right questions are asked from the start. Furthermore, as patients are not systematically asked whether the model of care used in their care delivery is what they want, MULTI-ACT can help the shift towards a model where patients are key stakeholders.
Closing remarks
Dr. René von Schomberg closed the conference summarising the key messages presented. He concluded by profiling the role MULTI-ACT could play in the implementation of multi-stakeholder initiatives within the Horizon Europe programme and how the project fits within the mission-oriented research objectives established at the European level, which include RRI elements.
Please visit the Documents & Multimedia sections of this website for more information on all presented during the Conference.


